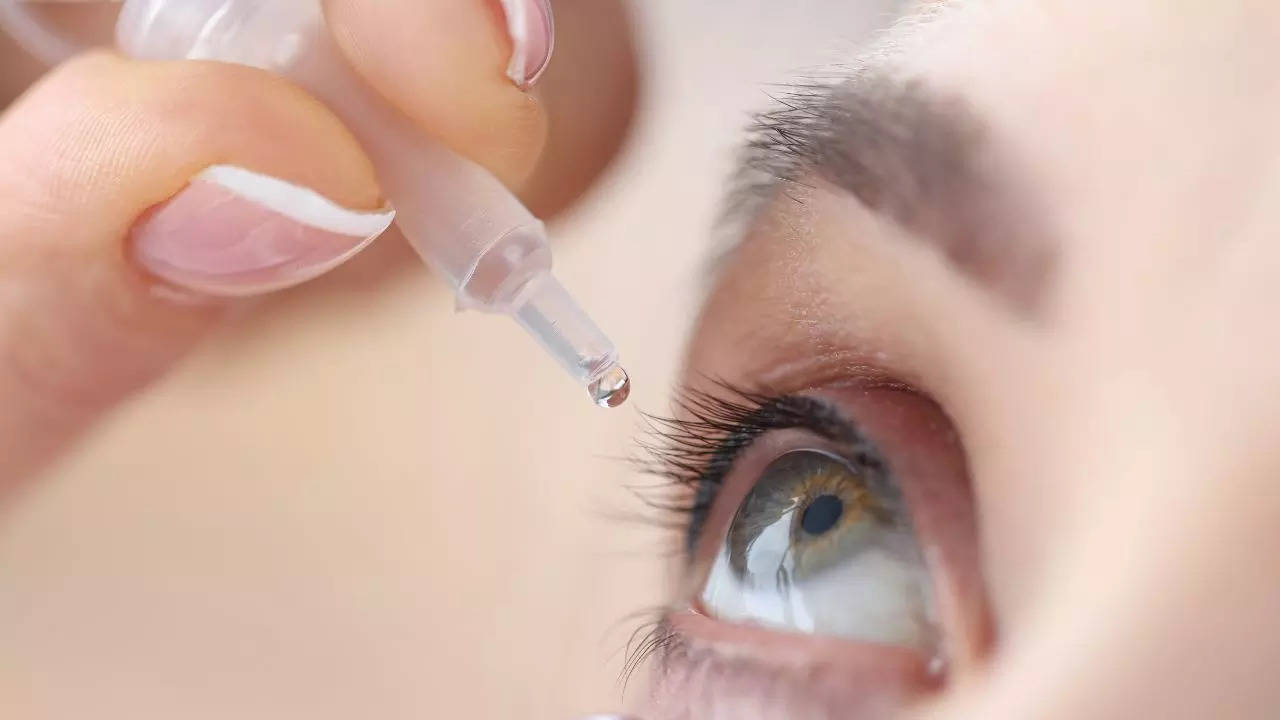
The first eye drop in India that can potentially replace reading glasses was launched on Tuesday. India’s drug regulatory agency, the Drugs Controller General of India (DCGI) approved the eye drop last month. The “PresVu” eye drop is developed by Entod Pharmaceuticals which is a Mumbai-based pharmaceutical firm and has 1.25 per cent pilocarpine hydrochloride. Pilocarpine is a plant-derived compound that has been used to treat various eye conditions and dry mouth and reduce eye pressure among other applications.
The eye drop treats presbyopia by reducing the size of the pupils, thereby enhancing the ability to see nearby objects. PresVu will be available from the first week of October and a single vial of the eye drop will cost Rs 345.
Speaking to ThePrint, Nikhil Masurkar, CEO of Entod Pharmaceuticals said that the eye drop’s launch in India is a result of extensive research that started in 2019. He added that an estimated 45 per cent of Indians above the age of 40 years have presbyopia.
Data from phase 3 clinical trials of the drug; carried out in 250 patients across 10 sites, and submitted to the DCGI showed that it works best in people aged 40 to 55 years with mild-to-intermediate presbyopia, temporarily correcting vision problems.
Masurkar explained that a single drop of PresVu begins to take effect in just 15 minutes, with the effects lasting for up to six hours. “If a second drop is applied within three to six hours of the first, the effect can be prolonged even further,” Masurkar said while speaking to News18.
The eye drop must be used only under prescription by an ophthalmologist. One drop of the drug should be put in each eye every day. The effect lasts about six hours.
An additional drop can be put in each eye three to hours after the first drop. This can potentially correct blurred vision for three more hours. The company also claims that the drug starts working within 15 minutes of application but complete benefits are likely to be visible after 15 days of use.
Dr Dhananjay Bakhle, a senior pharmacologist and advisor to Entod Pharmaceuticals, said that for patients with presbyopia, the eye drop offers a non-invasive option that can enhance near vision without the need for reading glasses. Dr Bakhe added, “Its rapid efficacy and safety profile, demonstrated in clinical trials, makes it a valuable addition to the treatment arsenal.”
Masurkar said that while the study results have yet to be published in a peer-reviewed journal, the company plans to conduct post-marketing surveillance at major eye-care centres across India, including the All India Institute of Medical Sciences (AIIMS) in New Delhi. “We are preparing for post-marketing surveillance to understand the different aspects of the medicine better.”
(With inputs from agencies)

